Glioblastoma and malignant glioma are the most common and deadliest primary brain cancers in adults. Treatment options for glioblastoma are limited.
The Stupp protocol has become the standard of care for the treatment of glioblastoma since its publication in 2005 and has led to significant improvements in survival. It consists of radiotherapy and concomitant chemotherapy with temozolomide, an alkylating agent. However, the Stupp protocol only allows a median median overall survival of 14.6 months against 12 months with radiotherapy alone.
The glioblastoma tumor microenvironment has been characterized as highly immunosuppressive. Treatments currently being explored to reverse the intrinsic immunosuppression of glioblastoma therefore include PD-1 inhibitors, autolytic vaccines, dendritic cell-based vaccines, and oncolytic viruses. Other glioblastoma treatments under investigation include different methods of radiation therapy (eg, photon intensity modulated, proton beam, and low-dose whole brain) in place of standard-dose radiation therapy and in combination with various agents targeted.
These different approaches are associated with systemic adverse events that have a negative impact on quality of life and are of limited interest in patients with poor functional status and/or multiple comorbidities.
Interest in TTFields therapy stems from its tolerable safety profile and longer operating time compared to the Stupp protocol. Treatment strategies in combination with TTFields provide significant clinical benefit while limiting additional toxicity in brain cancer patients and may extend to patients with other solid tumors.

Tumor Treating Fields (TTF) are a type of electromagnetic field therapy using low-intensity, intermediate-frequency electric fields to treat cancer. Novocure's TTF, also known as Optune Lua is approved in the US and EU for the treatment of glioblastoma (brain cancer).
About a year and a half after receiving FDA approval as a first-line treatment for mesothelioma, Novocure has obtained CE Mark for its Tumor Treating Fields therapy in Europe.
The NovoTTF-100L system will also be offered in combination with pemetrexed and platinum-based chemotherapy for the treatment of inoperable, advanced or metastatic malignant pleural mesothelioma, a rare lung cancer linked to asbestos exposure.
Malignant gliomas remain a difficult cancer to treat due to the limitations of therapeutic and effective options. In 2 large, randomized, controlled phase 3 trials, the addition of TTFields was associated with an increase in overall survival when combined with adjuvant chemotherapy with temozolomide (temozolomide) in patients with newly diagnosed glioblastoma (newly diagnosed glioblastoma) and comparable overall survival compared to standard chemotherapy in patients with recurrence. glioblastoma (rGBM).
TTFields target cancer cells through several mechanisms of action, including suppression of proliferation, migration and invasion, disruption of DNA repair and angiogenesis, antimitotic effects and induction of apoptosis and immunogenic cell death. Having multiple mechanisms of action makes TTFields an attractive modality to combine with standard, rescue and novel treatment regimens (e.g. radiation therapy, chemotherapy and immunotherapy). Treatment in the field of malignant gliomas is evolving to emphasize combinatorial approaches that work synergistically to improve patient outcomes. Here, we review the current use of TTFields in glioblastoma, discuss the mode of action and delivery of the treatment, and examine the potential for its wider adoption in other gliomas.
The incidence of gliomas, primary brain tumors originating from glial or neuronal precursor cells, increases with age, with higher rates among those 75 and older. High-grade malignant gliomas represent 35 to 45% of primary brain tumors. Due to its aggressive and diffusely infiltrating nature, recurrence is common, often resulting in rapid spread of the tumor to other regions of the brain, while the blood-brain barrier generally limits metastatic spread beyond the brain. Despite the expansion of treatment options for malignant gliomas in the decade since the approval of temozolomide,
long-term survival rates remain dismal.
From this daunting backdrop emerged Tumor Treatment Fields (TTFields), a novel locoregional antineoplastic treatment modality using low-intensity (1–3 V/cm), intermediate-frequency (~100–500 kHz) alternating electric fields, with a unique mechanism of action. TTField fields are delivered by 2 pairs of orthogonal transducer arrays. Matrix positioning is individualized to maximize therapeutic delivery of fields to the tumor bed, allowing for a targeted approach to minimize unwanted side effects.
In the Phase 3 EF-14 trial of newly diagnosed glioblastoma, TTFields plus maintenance temozolomide significantly improved median overall survival (median overall survival) compared to temozolomide alone. In the Phase 3 rGBM trial EF-11, TTFields monotherapy versus physicians' choice chemotherapy showed comparable survival profiles (6.6 months versus 6.0 months, respectively). Although these results may seem poor, at comparable results, the patients' quality of life was better with the TTFields option.
Based on these results, TTFields (200 kHz) are approved for adult patients with rGBM as monotherapy and for newly diagnosed glioblastoma in combination with adjuvant temozolomide in the United States, Europe, Japan, Israel and in Hong Kong, China. The National Comprehensive Cancer Network® guidelines recommend TTFields for newly diagnosed glioblastoma with Grade 1 evidence and rGBM with Grade 2B evidence [15] and the American Society of Clinical Oncology recognizes TTFields as a new device for the treatment of brain cancer.
Administration of the TTFields
In glioblastoma, TTFields (200 kHz) are delivered to the tumor bed non-invasively and continuously using the Optune® system through 2 pairs of orthogonal transducer arrays attached to the patient's shaved scalp according to the location of the tumor.
Optimal frequencies for maximizing cytotoxic effects vary by tumor type and may be inversely correlated with cell size. For a specific frequency, the minimum electrical intensity determining the degree of antimitotic cell death is about 1 V/cm. The electrical intensity reaching the target depends on the position of the tumor as well as the conductivity and impedance of the surrounding tissues. The positioning of the transducer array is individualized for each patient to ensure maximum field strength at the tumor bed, with arrays replaced a minimum of twice a week and, for glioblastoma, the scalp shaved to maintain optimal contact and operation.
Since it is not possible to measure the frequency and intensity of TTFields fields at the target site in patients, computer generated models/algorithms are used to simulate the expected trajectory and properties of the electric field in order to optimize the size and layout of networks. Realistic computer phantoms were generated to model the delivery of TTFields to brain and torso tumors.
The Novocure Patient Transducer Array Layout System (NovoTAL™) is FDA-cleared software that configures the optimal transducer array layouts based on the patient's head and tumor size and location, as determined by post-contrast T1 axial and coronal sequences of cerebral MRI. NovoTAL ensures that the field strength remains highest at the tumor bed and allows network adjustment to retarget tumors as they respond or progress.



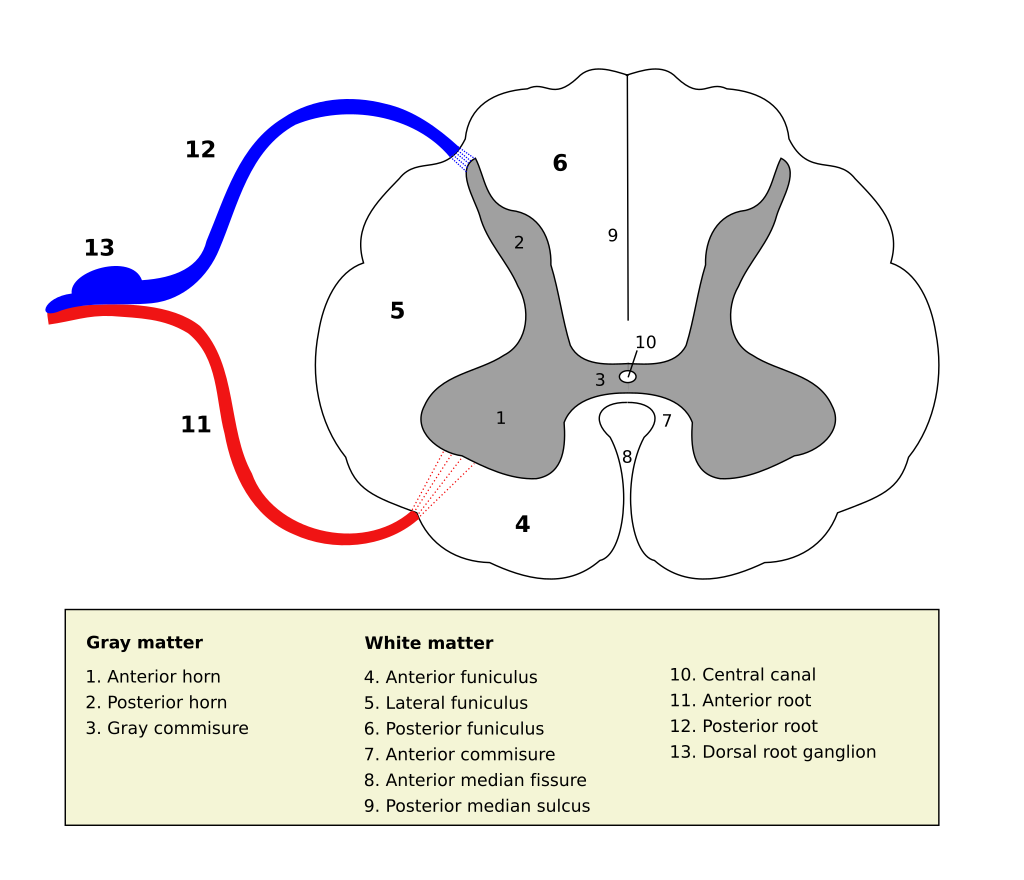 Source Wikipedia/User Polarlys
Source Wikipedia/User Polarlys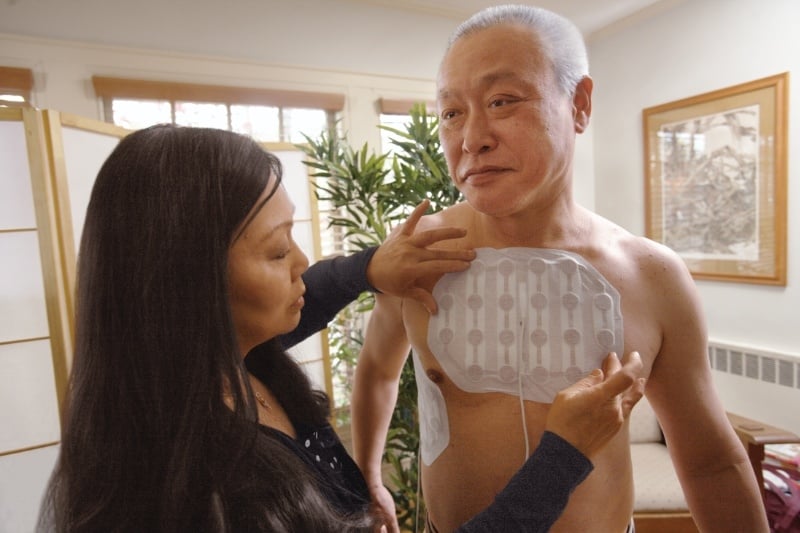
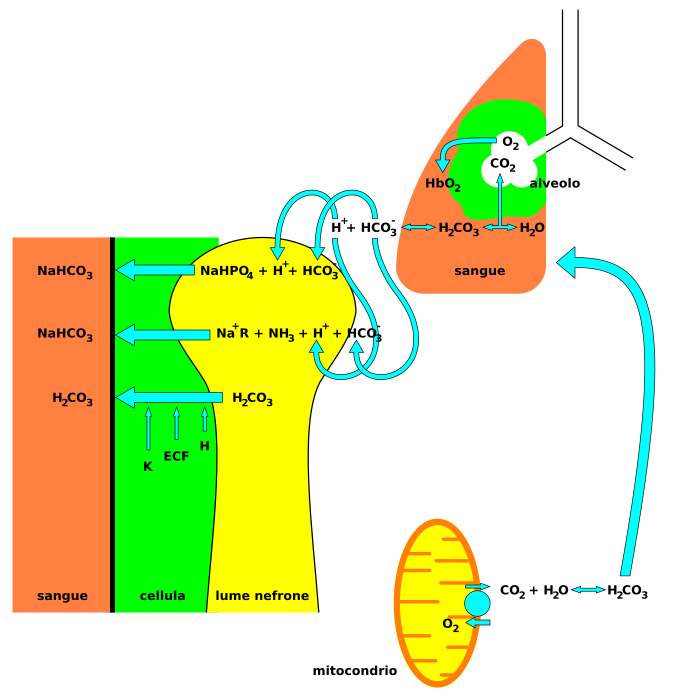 Nervous system involvement may be seen with acidosis. Signs and symptoms that may be seen in acidosis include headaches, confusion, feeling tired, tremors, sleepiness, flapping tremor, and dysfunction of the cerebrum of the brain which may progress to coma if there is no intervention.
Nervous system involvement may be seen with acidosis. Signs and symptoms that may be seen in acidosis include headaches, confusion, feeling tired, tremors, sleepiness, flapping tremor, and dysfunction of the cerebrum of the brain which may progress to coma if there is no intervention.