Numerous studies have suggested that the medical classification of many neurodegenerative diseases (Alzheimer's, ALS, Parkinson's, many dementias) is artificial because patients may have biomarkers involving up to four comorbidities.
The coexistence of amyotrophic lateral sclerosis (ALS) with clinical forms of Parkinson disease (PD), although uncommon, is found to a greater degree than one would expect by chance. The pathological mechanisms of ALS and Parkinson disease are still not understood, and the coexistence of these two diseases suggests that they could share mechanisms in common.
In this publication, authors from Colombia, Brazil, USA present a sample of patients with clinically definitive or probable ALS who were evaluated with single-photon emission computed tomography (SPECT/TRODAT) and compared with non-ALS controls. SPECT is a nuclear medicine tomographic imaging technique using gamma rays.
Dopamine is a neurotransmitter that modulates a variety of human functions such as motion, cognition, emotions, and the peristaltic reflexes in the gastrointestinal tract. The transport of this molecule at the neuron pre- and postsynaptic junctions is mediated by an axonal membrane dopamine transporter (DAT) that regulates dopamine levels within the synaptic cleft.
Development of various imaging ligands that specifically bind to DAT has been of interest to understand the functioning of these transporters and also to diagnose and monitor the treatment of Parkinson disease. TRODAT was shown to have a high sensitivity and specificity to measure the gradual loss of DAT in Parkinson disease patients.
Patients with clinically definite or probable ALS were assessed with the amyotrophic lateral sclerosis functional rating scale (ALSFRS) to define severity and had their demographic data collected. The TRODAT results of patients with ALS were compared with those of patients with a diagnosis of Parkinson disease with less than 10 years of duration, and with patients with a diagnosis of others movement disorders not associated with neurodegenerative diseases.
A total of 75% of patients with ALS had TRODAT results below the levels considered normal; that was also true for 25% of the patients in the control group without neurodegenerative disease, and for 100% of the patients in the Parkinson disease group. A statistically significant difference was found between patients with ALS and the control group without neurodegenerative disease in the TRODAT values < 0.05.
Conclusions: This study fits with the neuropathological and functional evidence that demonstrates the existence of nigrostriatal dysfunction in patients with ALS.
Sometimes ALS and Parkinson's are associated, for example in a unique neurodegenerative disease found on the island of Guam which is attributed to a toxin in cycad flour.
Progressive degeneration of functionally related groups of neurons occurs in certain infective, toxic, nutritional and genetically determined neurological diseases. It also takes place in normal aging, and several of the regions that undergo selective decay with the passage of time.
One (old) hypothesis that I like, is that features associated with Parkinson's disease, Alzheimer's disease, and ALS may be non-specific indicators of neuronal "disease", with certain morphological markers tending to appear more frequently in particular circumstances and particular regions associated with the pathology of particular diseases.


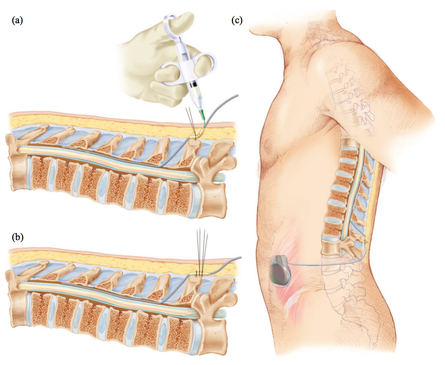 The imaging analyzes that this study will produce, will make it possible to define a subgroup of patients with Parkinson's disease who will have benefited from the treatment and will help to define rules about when using this therapy in order to avoid unnecessary interventions.
The imaging analyzes that this study will produce, will make it possible to define a subgroup of patients with Parkinson's disease who will have benefited from the treatment and will help to define rules about when using this therapy in order to avoid unnecessary interventions. Deep brain stimulation (DBS) is a neurosurgical procedure involving the placement of a medical device called a neurostimulator, which sends electrical impulses, via implanted electrodes, to specific targets in the brain (the cerebral nucleus) for treatment movement disorders, including Parkinson's disease. illness, essential tremor, dystonia, and other conditions such as obsessive-compulsive disorder (OCD) and epilepsy. Its underlying principles and mechanisms are not fully understood.
Deep brain stimulation (DBS) is a neurosurgical procedure involving the placement of a medical device called a neurostimulator, which sends electrical impulses, via implanted electrodes, to specific targets in the brain (the cerebral nucleus) for treatment movement disorders, including Parkinson's disease. illness, essential tremor, dystonia, and other conditions such as obsessive-compulsive disorder (OCD) and epilepsy. Its underlying principles and mechanisms are not fully understood. In this article, the authors outline the principles of drug selection for Parkinson disease prevention trials, focused on proof-of-concept opportunities that will help establish a methodological foundation for this fledgling field.
In this article, the authors outline the principles of drug selection for Parkinson disease prevention trials, focused on proof-of-concept opportunities that will help establish a methodological foundation for this fledgling field.