There are often many causes to a non-communicable disease, particularly neurodegenerative diseases are more a consequence of a systemic failure than caused by a specific phenomenon. The multitude of papers assigning a specific mechanism, each time different, to neurodegenerative diseases is just noise that drags down knowledge acquisition in these domains. Some authors have hinted at a phase transition to explain the misfolding of some proteins, but what triggers this phase transition was elusive.
In this post, I discuss a very general paper. https://elifesciences.org/reviewed-preprints/107962v1
In simple terms, the authors have discovered how our innate immune system launches an extremely powerful and rapid response to a tiny signal from a pathogen. This has implications for age-related diseases such as cancer or neurodegenerative diseases.
The Core Problem:
Our immune system needs to react decisively to a single bacterium or virus. This involves a massive cellular response like inflammation or programmed cell death (pyroptosis, apoptosis). However, the initial detection of a pathogen (a single molecule binding to a receptor) provides almost no energy to power this massive response.
The Discovery - "Metastable Supersaturation":
 The authors found that key immune signaling proteins, specifically those containing Death Fold Domains (DFDs) (like ASC, FADD, BCL10, MAVS, TRADD), exist in a unique physical state inside our cells called metastable supersaturation. These full-length adaptors retain nucleation barriers and are able to exist supersaturated in cells. In contrast, many receptors and effectors do not. This localizes the “spring-loaded” behaviour to central adaptors that link receptor sensing to downstream cell-fate decisions.
The authors found that key immune signaling proteins, specifically those containing Death Fold Domains (DFDs) (like ASC, FADD, BCL10, MAVS, TRADD), exist in a unique physical state inside our cells called metastable supersaturation. These full-length adaptors retain nucleation barriers and are able to exist supersaturated in cells. In contrast, many receptors and effectors do not. This localizes the “spring-loaded” behaviour to central adaptors that link receptor sensing to downstream cell-fate decisions.
A subset of death-fold domains (DFDs) are intrinsically “supersaturable.” Using a systematic screen of 109 human DFDs with a distributed amphifluoric FRET (DAmFRET) assay in yeast, the authors show that a minority of DFDs switch from soluble → assembled in a discontinuous (nucleation-limited) manner — the hallmark of a large intrinsic nucleation barrier. These discontinuous DFDs can therefore exist metastably above their saturation concentration (Csat) while remaining soluble (i.e. supersaturated).
Imagine a supersaturated solution of sugar water. It holds far more dissolved sugar than it should be able to. It remains liquid until you drop in a single sugar crystal, which instantly triggers the entire solution to crystallize.
Similarly, these DFD proteins are present in concentrations far higher than their natural solubility limit. They are kept in a soluble, "primed" state only by a high energy barrier that prevents them from spontaneously assembling (like the sugar needing a seed crystal).
This state acts as a long-term energy reservoir. The cell expends energy to produce and maintain these high levels of protein, storing potential energy for a future immune response. The authors show that tissues/cell types with shorter lifespans (e.g., monocytes) tend to express higher adaptor supersaturation than long-lived cells (neurons), suggesting a trade-off between rapid innate responsiveness and longevity. They also find conservation of nucleation barriers in distant taxa (fish, sponges, bacteria), indicating the mechanism is ancient.
How It Works for Immunity: When a pathogen is detected (the initial signal), the pathogen-bound receptor acts as the "seed crystal." This seed triggers the instantaneous, explosive polymerization of the supersaturated adaptor proteins (like ASC or FADD). This amplification process consumes the stored energy from supersaturation, converting it into a massive biochemical signal that leads to inflammation or cell death.
This allows for a response that is immediate, decisive, and independent of the cell's current metabolic energy (which is often hijacked by pathogens).
The Trade-Off is Immunity vs. Longevity:
This mechanism comes with a cost. Maintaining a supersaturated, "primed" state means there's always a risk of a spontaneous, accidental activation (a stochastic nucleation event). This would lead to unwanted inflammation or cell death without any infection. The authors found evidence that this trade-off is real: short-lived immune cells (like monocytes) have much higher levels of supersaturation than long-lived cells (like neurons). This suggests a fundamental thermodynamic drive where the need for strong immunity may inherently limit a cell's lifespan.
The authors also showed this system is highly specific (DFDs from one pathway don't accidentally trigger others) and that the mechanism is evolutionarily ancient, found in everything from humans to sponges to bacteria, indicating its fundamental importance.
This groundbreaking discovery opens up entirely new avenues for treating a wide range of diseases by targeting this "supersaturation engine."
Autoinflammatory and Autoimmune Diseases Examples: Crohn's disease, rheumatoid arthritis, lupus, CAPS (Cryopyrin-Associated Periodic Syndromes), type 1 diabetes.
Infectious Diseases Examples: Sepsis, severe viral infections (e.g., COVID-19, flu).
Cancer Application: Some cancers evade the immune system by preventing immune cells from initiating cell death (apoptosis) in cancerous cells. They might do this by interfering with the supersaturation or nucleation of proteins like FADD.
Neurodegenerative Diseases Examples: Alzheimer's, Parkinson's, ALS.
Therapeutic Strategy: This research provides a deeper biophysical understanding of how proteins form aggregates. Insights into controlling nucleation barriers could lead to strategies for preventing the initial "seed" event that sparks the catastrophic aggregation of proteins like amyloid-beta or alpha-synuclein.
Risks, trade-offs, and practical challenges
Immunity vs longevity trade-off. The authors argue a thermodynamic tradeoff: lowering supersaturation protects cells from spontaneous death but reduces rapid responsiveness to pathogens. Therapies that blunt supersaturation may increase infection susceptibility.
Off-target/cross-seeding risk. Although the interactome is relatively specific, some cross-nucleation exists (e.g., PYD↔DED). Inhibiting one adaptor could have unintended effects on other pathways, or conversely, seeding one adaptor therapeutically could accidentally trigger another.
Drugging interfaces is hard. Filamentizing interfaces and nucleation kinetics are complex to target with small molecules; biologics or degradation approaches may be more tractable but have delivery challenges.
Temporal and quantitative control required. Because the system is switch-like, small quantitative changes in concentration or barrier height can produce large outcome differences; therapies need tight control to avoid tipping the balance toward immunodeficiency or hyperinflammation.
In conclusion This study moves beyond simply listing the components of immune pathways to explaining the fundamental physics and energy dynamics that make them work. By understanding that immunity is powered by a "loaded spring" mechanism of metastable supersaturation, we can now think about designing much smarter, more precise drugs that either stabilize this spring (for autoimmune diseases) or trigger it on command (for cancer).

 What did the researchers do?
Nine individuals with Parkinson’s, all of whom had deep brain stimulation (DBS) implants, participated in up to 12 cycling sessions over a month. These implants allowed researchers to directly record brain activity from a small structure deep in the brain called the subthalamic nucleus (STN)—a region strongly involved in movement control and a common target in Parkinson’s treatments.
What did the researchers do?
Nine individuals with Parkinson’s, all of whom had deep brain stimulation (DBS) implants, participated in up to 12 cycling sessions over a month. These implants allowed researchers to directly record brain activity from a small structure deep in the brain called the subthalamic nucleus (STN)—a region strongly involved in movement control and a common target in Parkinson’s treatments. The authors note that, although their findings are exploratory and cannot be directly applied clinically yet, the identified drugs could be considered for future clinical trials. Indeed, funding should be sought for these future trials, and since it is almost always private investors who finance clinical trials, they would require more information before making any commitments.
The authors note that, although their findings are exploratory and cannot be directly applied clinically yet, the identified drugs could be considered for future clinical trials. Indeed, funding should be sought for these future trials, and since it is almost always private investors who finance clinical trials, they would require more information before making any commitments.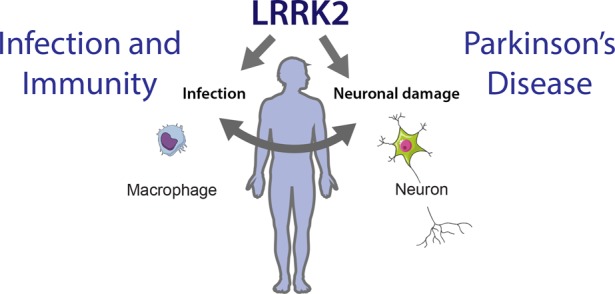
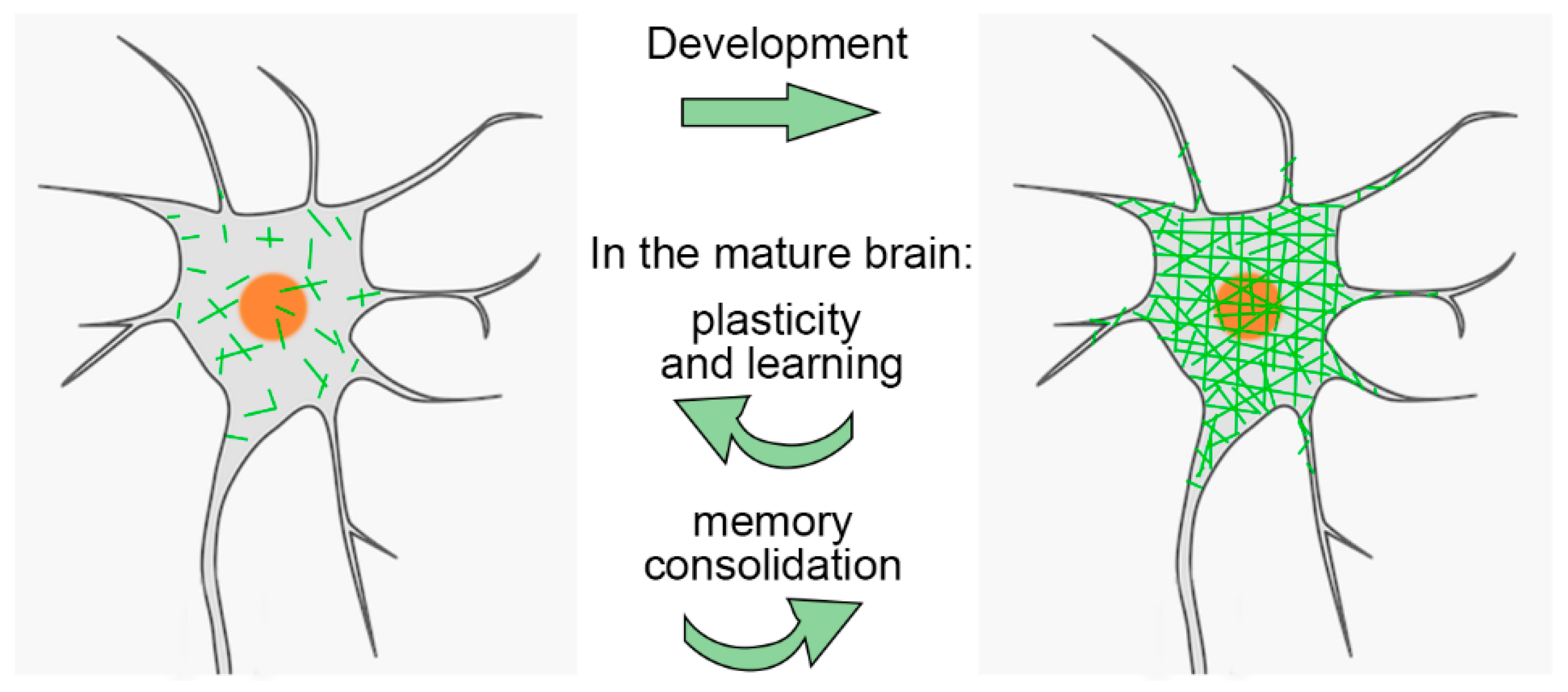 PNNs are especially important during brain development. They help close the "critical period" of heightened plasticity in childhood. Interestingly, while PNNs are degraded in adults, this plasticity can be partially restored. For example, PNN removal can promote recovery in stroke models
PNNs are especially important during brain development. They help close the "critical period" of heightened plasticity in childhood. Interestingly, while PNNs are degraded in adults, this plasticity can be partially restored. For example, PNN removal can promote recovery in stroke models This study investigates whether acupuncture has any impact on long-term health outcomes, such as mortality, disease progression, or complications, in individuals newly diagnosed with Parkinson’s disease (PD) in South Korea. Indeed, it is unclear what constitutes an effective acupuncture session for Parkinson's disease, and individuals interested should receive at least one session every two months. It is commonly believed that in PD, reduced mobility due to tremors, postural imbalance, and rigidity likely contributes to poor circulation and decreased gastrointestinal motility, leading to bowel obstructions and impaired swallowing, which can, in turn, result in recurrent aspiration pneumonia. The benefits may arise from the fact that people with PD might find it easier to move.
This study investigates whether acupuncture has any impact on long-term health outcomes, such as mortality, disease progression, or complications, in individuals newly diagnosed with Parkinson’s disease (PD) in South Korea. Indeed, it is unclear what constitutes an effective acupuncture session for Parkinson's disease, and individuals interested should receive at least one session every two months. It is commonly believed that in PD, reduced mobility due to tremors, postural imbalance, and rigidity likely contributes to poor circulation and decreased gastrointestinal motility, leading to bowel obstructions and impaired swallowing, which can, in turn, result in recurrent aspiration pneumonia. The benefits may arise from the fact that people with PD might find it easier to move.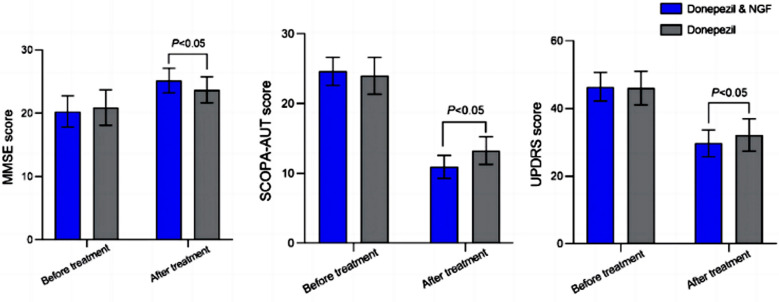 The authors focused on adiponectin, an adipocytokine, i.e. a molecule produced by adipose tissue, which is involved, among other things, in the regulation of lipid and glucose metabolism. Adiponectin modulates inflammatory cascades by modifying the action and production of inflammatory cytokines, but the link between adiponectin and Parkinson's disease is not obvious unless we consider that Parkinson's disease is due to a metabolic disorder. The relationship with the soluble tumor necrosis factor receptor (sTNFR) is even less obvious. Nothing in the article explains why these two molecules were studied.
The authors focused on adiponectin, an adipocytokine, i.e. a molecule produced by adipose tissue, which is involved, among other things, in the regulation of lipid and glucose metabolism. Adiponectin modulates inflammatory cascades by modifying the action and production of inflammatory cytokines, but the link between adiponectin and Parkinson's disease is not obvious unless we consider that Parkinson's disease is due to a metabolic disorder. The relationship with the soluble tumor necrosis factor receptor (sTNFR) is even less obvious. Nothing in the article explains why these two molecules were studied.