ApoE Isoforms, Glucose and Lipid Metabolism, and PPARα in Neurodegenerative Diseases
Apolipoprotein E (apoE) is essential for lipid transport and neuronal repair in the central nervous system. Of its three main forms—apoE2, apoE3, and apoE4—apoE3 is considered the “neutral” variant, supporting normal lipid balance and synaptic stability. In contrast, apoE4 is a major genetic risk factor for late-onset Alzheimer’s disease and several other neurodegenerative disorders.
Structural differences between apoE3 and apoE4 change how they bind lipids and trigger a cascade of metabolic and inflammatory disturbances that weaken neurons over time.
A key part of this vulnerability lies in energy metabolism. Neurons rely heavily on glucose, but in aging or disease, glucose uptake and utilization often decline. ApoE4 has been linked to reduced glucose transport and impaired mitochondrial efficiency, leading to energy shortages and oxidative stress.
The peroxisome proliferator-activated receptor alpha (PPARα), a nuclear receptor regulating genes involved in fatty acid oxidation and lipid balance, plays a protective role in this context.
Together, apoE isoforms, glucose metabolism, lipid regulation, and PPARα signaling form a tightly linked metabolic network that shapes the progression of neurodegenerative diseases.
The New Study: Sortilin, ApoE, and Neuronal Energy Metabolism
A recent study explored how sortilin, a neuronal receptor, interacts with apoE3 and apoE4 to regulate how neurons use fatty acids for energy. Sortilin is known to participate in brain lipid metabolism and is thought to cooperate with apoE3 to maintain healthy neuronal lipid processing. The authors hypothesized that this partnership fails when sortilin is absent or when apoE4 replaces apoE3.
Experimental Models
Researchers examined four types of genetically modified mice:
- E3WT: human apoE3, sortilin present
- E3KO: human apoE3, sortilin knockout
- E4WT: human apoE4, sortilin present (but functionally impaired)
- E4KO: human apoE4, sortilin knockout
Key Findings
Only the E3WT neurons displayed high mitochondrial respiration (oxygen consumption). All other groups—E3KO, E4WT, and E4KO—showed lower respiration, even though their mitochondria were structurally normal. ➡️ Interpretation: Sortilin–apoE3 interaction is required for neurons to reach full mitochondrial energy capacity.
To identify which energy pathways were affected, the researchers blocked specific fuel routes:
UK5099 blocked glucose-derived pyruvate entry into mitochondria.
Etomoxir blocked long-chain fatty acid (LCFA) import via CPT1A.
They found that the defect was specific to long-chain fatty acid metabolism. Neurons could still metabolize medium- and short-chain fatty acids, which enter mitochondria independently of the carnitine transport system.
Human Cell Models
Using human induced pluripotent stem cells (iPSCs) carrying the same genetic combinations, the team generated astrocytes and neurons. All appeared normal structurally, but only E3WT neurons used both glucose and LCFAs for energy. The others (E3KO, E4WT, E4KO) relied exclusively on glucose, mirroring the mouse results.
When E4 neurons were cultured in medium from E3 astrocytes, their ability to use LCFAs returned. ➡️ Interpretation: ApoE4 disrupts sortilin’s metabolic function, but factors secreted by apoE3 astrocytes can restore it.
Neuronal Activity
Electrical recordings showed that under normal glucose conditions, E3WT and E3KO neurons fired similarly. When glucose was scarce, E3WT neurons maintained their activity by switching to fatty acid metabolism, while E3KO neurons did not. ➡️ Interpretation: Sortilin enables neurons to use fatty acids as an alternative fuel, a key mechanism for metabolic resilience during glucose shortage.
Pharmacological Rescue
Treatment with bezafibrate, a PPARα agonist, restored PPARα activity and increased expression of CPT1A in E3KO and E4 neurons. This also reinstated their sensitivity to etomoxir, indicating that fatty acid oxidation had resumed. ➡️ Interpretation: Activating PPARα can compensate for metabolic defects caused by apoE4 or loss of sortilin.
Conceptual Model
According to the authors, sortilin and apoE3 work together to import and metabolize lipids (especially polyunsaturated and long-chain fatty acids) and to activate PPARα-dependent genes for energy production and neuroprotection.
ApoE4, by binding sortilin abnormally, mimics a loss of sortilin function. Without this partnership, neurons lose their ability to oxidize long-chain fatty acids, leading to reduced mitochondrial respiration, lower levels of protective lipids, and weaker PPARα activation — ultimately impairing neuronal energy resilience.
Translational Outlook
These results are mechanistically insightful but not yet directly applicable to humans. The models reveal how apoE4 and sortilin influence neuronal metabolism, yet human validation remains essential before any clinical translation.
In neurodegenerative diseases, timing is critical. Because neurons in the adult brain do not divide and have very limited regenerative capacity, much of the damage is already irreversible once symptoms appear. Consequently, such studies are most valuable for developing preventive or early interventions, rather than curative therapies.
Current research, therefore, focuses on:
identifying individuals at risk (e.g., through APOE genotyping or early metabolic biomarkers),
and evaluating whether long-term activation of protective pathways — such as PPARα or mitochondrial support mechanisms — could maintain neuronal energy balance and delay disease onset.
Even if started later in life, treatments that restore lipid metabolism or support mitochondrial function might still preserve remaining neurons and slow disease progression.


 By Manu5 - http://www.scientificanimations.com/wiki-images/
By Manu5 - http://www.scientificanimations.com/wiki-images/ Ils ont confirmé qu'une exposition prolongée aux NRTI était associée à un risque moindre de développer la maladie d’Alzheimer.
- D’après les données du VA, chaque année supplémentaire de traitement par NRTI était associée à une réduction de 4 à 6 % du risque de maladie d’Alzheimer.
- D’après les données de MarketScan, la réduction était encore plus marquée : de 10 à 13 % par année d’utilisation.
Ils ont confirmé qu'une exposition prolongée aux NRTI était associée à un risque moindre de développer la maladie d’Alzheimer.
- D’après les données du VA, chaque année supplémentaire de traitement par NRTI était associée à une réduction de 4 à 6 % du risque de maladie d’Alzheimer.
- D’après les données de MarketScan, la réduction était encore plus marquée : de 10 à 13 % par année d’utilisation.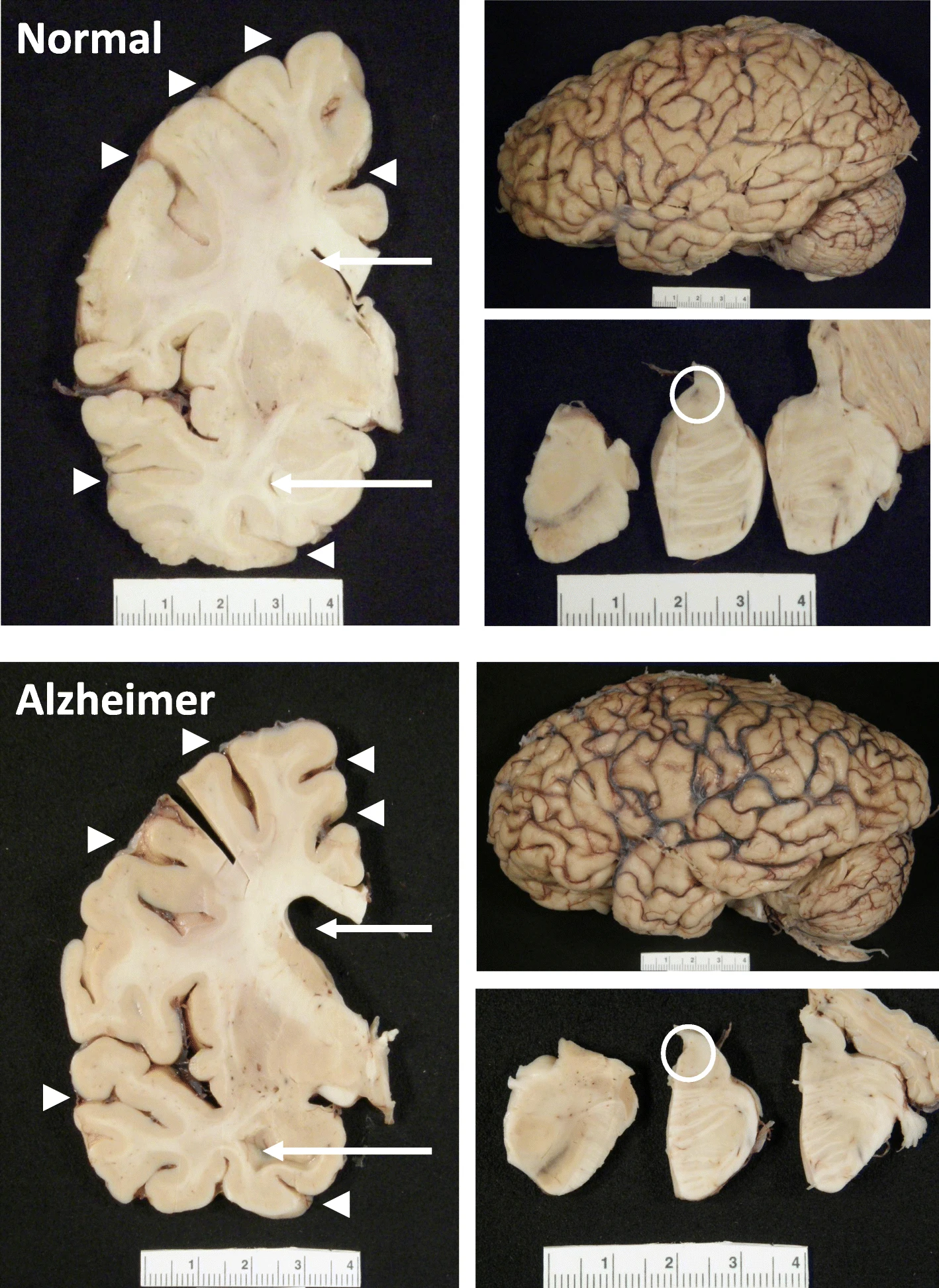 The hippocampus, a small, seahorse-shaped structure buried deep within the brain, is best known for its role in memory formation and learning. It is an exceptionally vulnerable structure, with perfusion deficits often observed in diseases related to learning and memory. However, a brain affected by Alzheimer's disease tends to exhibit at least moderate cortical atrophy, including in the precuneus and posterior cingulate gyrus. It should be noted that the posterior cingulate gyrus is adjacent to the hippocampus.
The hippocampus, a small, seahorse-shaped structure buried deep within the brain, is best known for its role in memory formation and learning. It is an exceptionally vulnerable structure, with perfusion deficits often observed in diseases related to learning and memory. However, a brain affected by Alzheimer's disease tends to exhibit at least moderate cortical atrophy, including in the precuneus and posterior cingulate gyrus. It should be noted that the posterior cingulate gyrus is adjacent to the hippocampus.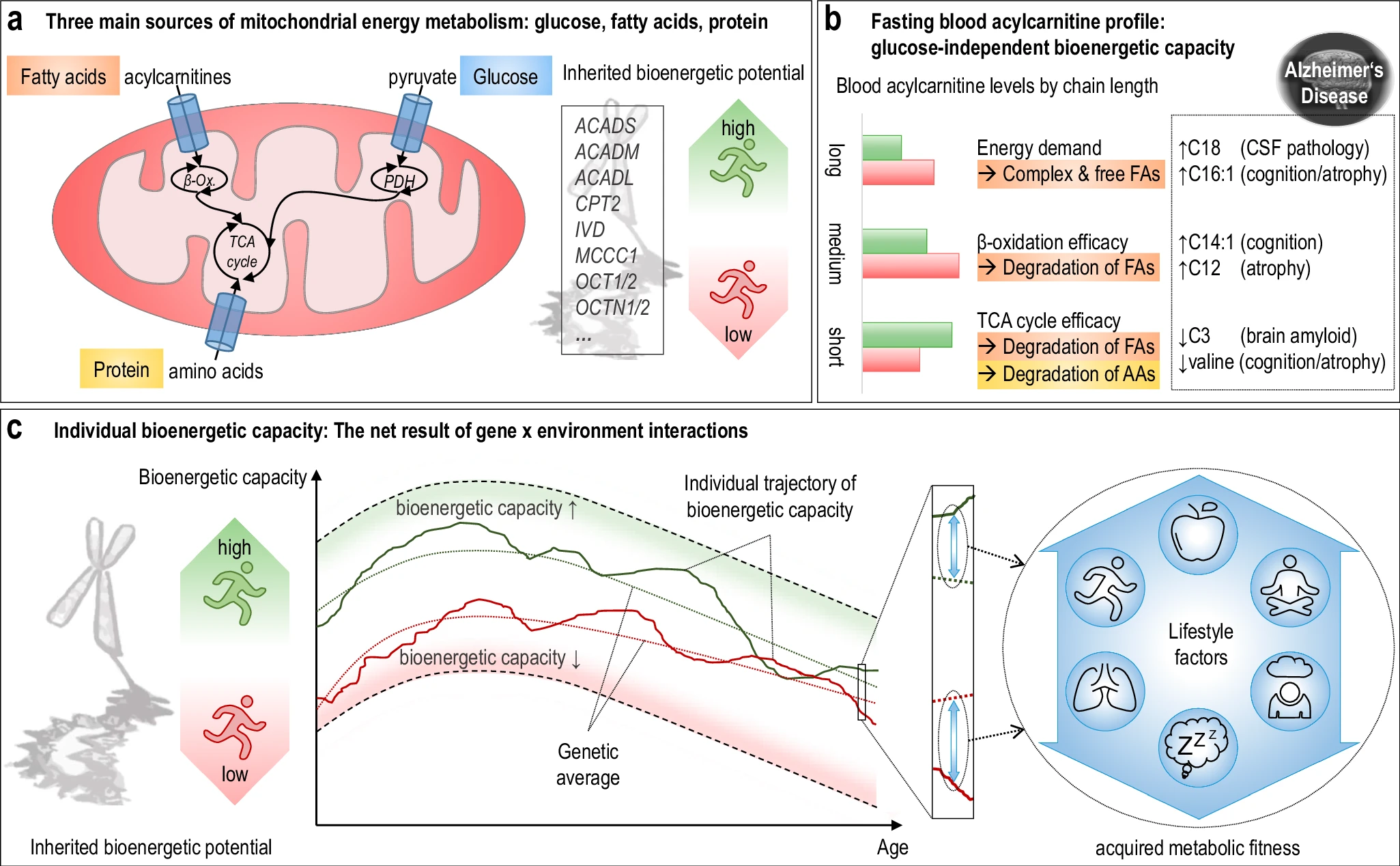 The researchers used acylcarnitine profiles from blood samples to identify distinct bioenergetic subgroups in Alzheimer's Disease (AD) patients and evaluate how bioenergetic capacity relates to disease progression.
They used data from 1,531 participants in the Alzheimer's Disease Neuroimaging Initiative (ADNI), and identified several bioenergetic subgroups with significant differences in AD biomarkers, cognitive function, and brain glucose metabolism.
These subgroups were primarily determined by modifiable factors (40-60%) related to beta-oxidation function, rather than genetic factors, suggesting potential for intervention.
The researchers used acylcarnitine profiles from blood samples to identify distinct bioenergetic subgroups in Alzheimer's Disease (AD) patients and evaluate how bioenergetic capacity relates to disease progression.
They used data from 1,531 participants in the Alzheimer's Disease Neuroimaging Initiative (ADNI), and identified several bioenergetic subgroups with significant differences in AD biomarkers, cognitive function, and brain glucose metabolism.
These subgroups were primarily determined by modifiable factors (40-60%) related to beta-oxidation function, rather than genetic factors, suggesting potential for intervention. Alzheimer's disease research has produced many hypotheses over the years, including cholinergic, inflammatory, viral, mitochondrial, tau, and amyloid. However, none of these hypotheses have led to treatments that can stop or reverse the disease. This leads to a search for new theories to explain these failures. But this may be because interventions occur too late in the disease progression, with brain damage irreparable and compensatory mechanisms saturated.
Alzheimer's disease research has produced many hypotheses over the years, including cholinergic, inflammatory, viral, mitochondrial, tau, and amyloid. However, none of these hypotheses have led to treatments that can stop or reverse the disease. This leads to a search for new theories to explain these failures. But this may be because interventions occur too late in the disease progression, with brain damage irreparable and compensatory mechanisms saturated.