A recent publication provides a detailed overview of recent advances and challenges in the development of RNA therapies for neurodegenerative diseases, particularly for amyotrophic lateral sclerosis (ALS).
 Early immunotherapies targeting amyloid-β in Alzheimer's disease initially showed reductions in amyloid plaques but failed to prevent cognitive decline. The FDA recently approved lecanemab as the first disease-modifying drug for Alzheimer's disease. However, its benefits appear to be limited to the early stages of the disease, as it does not stop neurodegeneration or improve cognition. This highlights the difficulty of modifying neurodegenerative diseases, which often progress despite treatment.
Early immunotherapies targeting amyloid-β in Alzheimer's disease initially showed reductions in amyloid plaques but failed to prevent cognitive decline. The FDA recently approved lecanemab as the first disease-modifying drug for Alzheimer's disease. However, its benefits appear to be limited to the early stages of the disease, as it does not stop neurodegeneration or improve cognition. This highlights the difficulty of modifying neurodegenerative diseases, which often progress despite treatment.
Most cases of ALS are recognized as proteinopathies involving RNA dysregulation, but in others, such as those with mutations in superoxide dismutase 1 (SOD1), these abnormalities are absent, suggesting different pathogenetic pathways.
Nucleic acid-based therapies (NATs) represent an emerging treatment class that targets RNA rather than proteins. These agents act by degrading disease-associated mRNA or altering translation processes. For example, ASOs inhibit translation by disrupting ribosome assembly or degrading mRNA.
Although ASO approaches are both reasonable and scientifically sound, discernible clinical benefit has not always been observed in humans.
Three clinical trials using ASOs to reduce huntingtin (HTT) protein in Huntington’s disease have been halted because the therapies did not consistently reduce mutant HTT levels or improve clinical outcomes. A trial of one ASO, tominersen, caused unexpected worsening in patients who received higher doses. This has raised concerns about potential ASO toxicity due to off-target effects, immune responses, or loss of normal HTT function, which is essential for cellular health.
ASOs targeting toxic genes in ALS, such as TDP-43 in ALS, have shown promise in preclinical studies in extending life and restoring motor function in animal models. Yet, inhibitions of SOD1 and C9orf72 in mice show motor deficits and memory impairment over time, warning researchers of the potential risks of long-term ASO treatments. Clinical trials, such as the Phase III study of tofersen (an ASO targeting SOD1), have demonstrated a reduction in SOD1 mRNA, although clinical (i.e. visible) benefit has not been established.
Results from a Phase III trial of the ASO tofersen, which targets SOD1 mRNA in ALS, were reported in 2022. In this study, a total of 108 participants were enrolled, 60 of whom were classified in the faster progression subgroup. Approximately 7% of participants receiving tofersen experienced serious neurological adverse events, including myelitis, chemical or aseptic meningitis, and lumbar radiculopathy, but the pharmaceutical and medical community has highlighted the case of a Swedish patient who saw real benefit from his therapy.
A new study, ATLAS (NCT04856982), aims to test tofersen in asymptomatic, and some are thought to be presymptomatic, carriers of the SOD1 mutation to determine whether early intervention can delay the onset of ALS.
ASO-mediated inactivation in neurodegenerative diseases faces major challenges. By the time symptoms of ALS appear, motor neuron damage may be too advanced. Symptoms appear because compensatory mechanisms are exhausted. This is similar to Parkinson’s disease, where symptoms only appear after significant neuronal loss.
It is also likely that current ASOs are not very selective, and may reduce the production of essential proteins, causing side effects. Targeting mutations or aberrant transcripts specifically with more selective ASOs, such as those used in the PRECISION-HD trials, could help preserve healthy protein function.
Identifying sensitive biomarkers, such as elevated plasma TDP-43 or cryptic HDGFL2 levels, could help diagnose ALS before symptoms appear. This could allow for timely therapeutic interventions to slow or prevent neurodegeneration before it becomes irreversible.
In summary, RNA-based therapies such as ASOs offer hope for neurodegenerative diseases but face significant challenges.

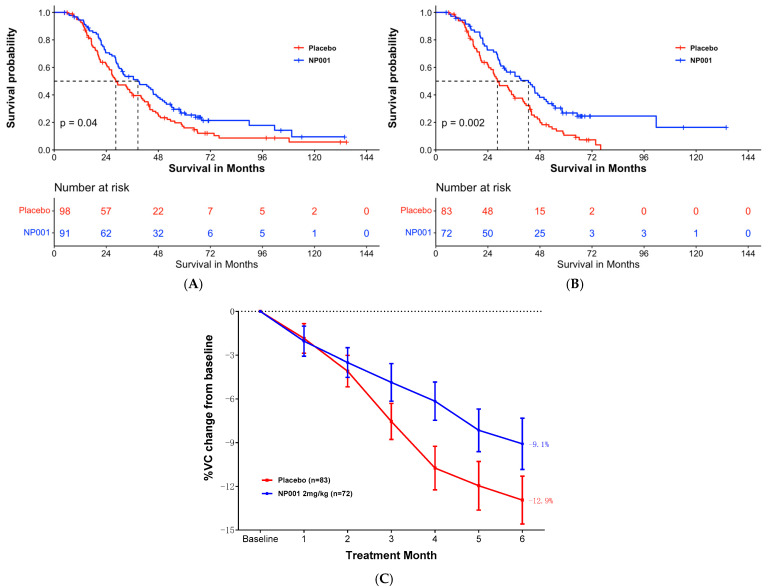 For example, the authors claim that survival of ALS patients with inflammation is 16 months longer than in the placebo arm. At 72 months there were only 3 people in the inflammation subset and two people in the placebo arm. Nobody can say anything about these numbers. If we use the same criteria, it shows that NP001 worsened the condition in all pALS with respect to the placebo branch, as starting from 72 months there were fewer survivors in the NP001 arm than in the placebo.
For example, the authors claim that survival of ALS patients with inflammation is 16 months longer than in the placebo arm. At 72 months there were only 3 people in the inflammation subset and two people in the placebo arm. Nobody can say anything about these numbers. If we use the same criteria, it shows that NP001 worsened the condition in all pALS with respect to the placebo branch, as starting from 72 months there were fewer survivors in the NP001 arm than in the placebo. The authors observed that, despite a decrease in limb motor functions, the feeding function of these mice was preserved until the late stages of the disease. Remarkably, the masseter muscle showed no reduction in muscle volume, wet weight, or muscle fiber cross-sectional area. Furthermore, no changes were observed in muscle fiber types, indicating a possible resistance of the masseter muscle to ALS-induced impairment. A potential reason for the lack of atrophy in the masseter muscle could be its higher number of muscle satellite cells compared to that of the gastrocnemius muscle. This abundance may promote the maintenance of muscle fiber nuclei, thereby contributing to muscle tissue regeneration.
The authors observed that, despite a decrease in limb motor functions, the feeding function of these mice was preserved until the late stages of the disease. Remarkably, the masseter muscle showed no reduction in muscle volume, wet weight, or muscle fiber cross-sectional area. Furthermore, no changes were observed in muscle fiber types, indicating a possible resistance of the masseter muscle to ALS-induced impairment. A potential reason for the lack of atrophy in the masseter muscle could be its higher number of muscle satellite cells compared to that of the gastrocnemius muscle. This abundance may promote the maintenance of muscle fiber nuclei, thereby contributing to muscle tissue regeneration. Two of them were phase III clinical trials, the others were phase II, so it cost probably $50M overall.
Two of them were phase III clinical trials, the others were phase II, so it cost probably $50M overall. Si dans la SLA/Maladie de Charcot les neurones moteurs meurent (ce dont je ne suis pas sûr
Si dans la SLA/Maladie de Charcot les neurones moteurs meurent (ce dont je ne suis pas sûr  C’est un progrès conséquent de l’état de l’art. Par contre ce neurone moteur ne s’est pas connecté via des synapses à d’autres cellules comme d’autres neurones moteurs, des interneurones ou des cellules de fibres musculaires. Il ne s’agit que d’un seul neurone, très court, pas des milliers de neurones moteurs long jusqu'à un mètre, qui composent la moelle spinale. De plus des neurones qui ne seraient pas accompagnés par une multitude de cellules non-neuronales (astrocytes, oligodendrocytes, etc..), mourraient rapidement. Enfin dans la moelle spinale il n’y a pas que des neurones moteurs.
C’est un progrès conséquent de l’état de l’art. Par contre ce neurone moteur ne s’est pas connecté via des synapses à d’autres cellules comme d’autres neurones moteurs, des interneurones ou des cellules de fibres musculaires. Il ne s’agit que d’un seul neurone, très court, pas des milliers de neurones moteurs long jusqu'à un mètre, qui composent la moelle spinale. De plus des neurones qui ne seraient pas accompagnés par une multitude de cellules non-neuronales (astrocytes, oligodendrocytes, etc..), mourraient rapidement. Enfin dans la moelle spinale il n’y a pas que des neurones moteurs. Il y a aussi quelque chose de fondamentalement contre-intuitif, voire obscène, dans l'idée que pour ralentir la progression d'une maladie caractérisée principalement par la perte intense de masse musculaire, il faudrait faire davantage d’exercice physique. Pourtant on sait que chez des sujets sains l'absence de mouvement pendant quelques jours à la suite d'un alitement, fait fondre spectaculairement la masse musculaire et qu'il faut des semaines pour retrouver le niveau antérieur de masse musculaire.
Il y a aussi quelque chose de fondamentalement contre-intuitif, voire obscène, dans l'idée que pour ralentir la progression d'une maladie caractérisée principalement par la perte intense de masse musculaire, il faudrait faire davantage d’exercice physique. Pourtant on sait que chez des sujets sains l'absence de mouvement pendant quelques jours à la suite d'un alitement, fait fondre spectaculairement la masse musculaire et qu'il faut des semaines pour retrouver le niveau antérieur de masse musculaire.