Adult-onset diabetes is a known risk factor for cognitive impairment and dementia, yet the study of diabetes in young people has been neglected until now.
The average age of onset of diabetes in adults is ~46 years, and roughly thirty years later neurodegenerative diseases may appear. People with early-onset diabetes when they reach the age of 46 years, have lived with their disease for at least 30 years. It is therefore possible that early-onset diabetes leads to early-onset dementia.
A recent prospective population-based cohort in the United Kingdom did find that a younger age at the onset of diabetes corresponded to a younger age at the onset of dementia, but this study did not specifically look at people with early diabetes. A new study on this subject indicates that people with early-onset diabetes are at significant risk of prematurely developing cognitive impairment and dementia with possible neuropathology.
 This study aimed to explore neurodegenerative disease biomarkers in cohort-derived biomarker banks as changes in key plasma biomarkers between the time of diabetes diagnosis and early adulthood have been correlated with worsening cognitive function in young adults with early-onset diabetes.
This study aimed to explore neurodegenerative disease biomarkers in cohort-derived biomarker banks as changes in key plasma biomarkers between the time of diabetes diagnosis and early adulthood have been correlated with worsening cognitive function in young adults with early-onset diabetes.
Participants with youth-onset diabetes (age of onset less than 20 years) were found in the SEARCH for Diabetes in Youth study, a multicenter population-based registry and cohort. A randomly selected subset of 50 SEARCH participants (n=25 type 1 diabetics, n=25 type 2 diabetics) was identified for inclusion in the plasma biomarker analysis.
Among SEARCH participants eligible for plasma biomarker analyses, the authors recruited and enrolled a subset of the Colorado SEARCH clinic site to complete positron emission tomography (PET) imaging to measure plasma accumulation amyloid and tau density in brain regions susceptible to Alzheimer's disease.
For their study of plasma biomarkers of neurodegeneration, scientists identified age-matched controls without diabetes from two cohorts with plasma samples stored to include adolescent controls from the Exploring Perinatal Study Outcomes in Children (EPOCH) (n = 25) and young adult controls from the CROCODILE (Control of Renal Oxygen Consumption, Mitochondrial Dysfunction and Insulin Resistance) study (n = 21).
The authors also recruited and enrolled a group of young adult controls from the University of Colorado Anschutz Medical Campus to complete PET imaging for amyloid density and tau in brain regions susceptible to Alzheimer's disease.
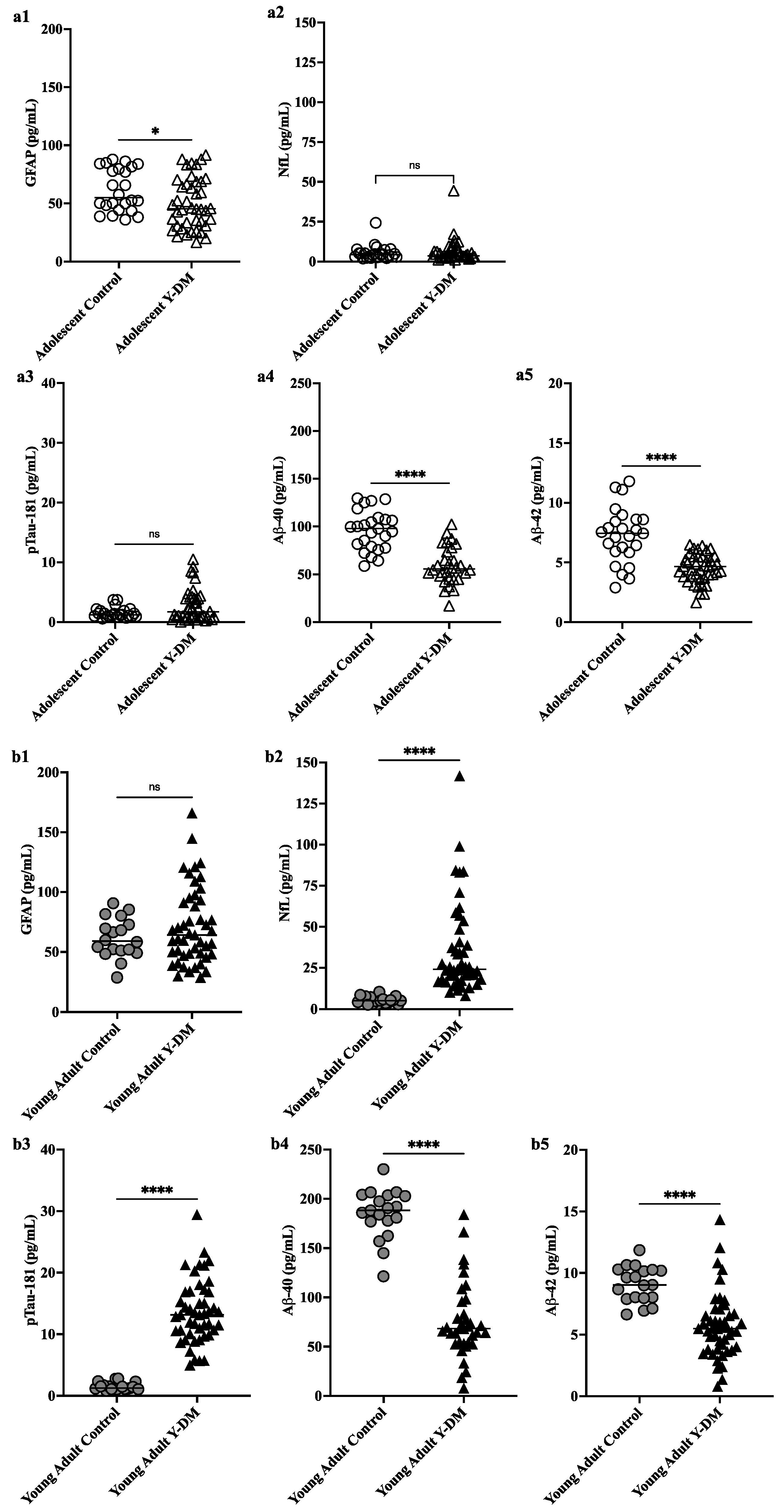
By studying these two types of biomarkers (plasma and molecular imaging), scientists found evidence of potentially greater neuropathology of neurodegenerative diseases in young adults with early-onset diabetes, where plasma pTau181 was significantly higher and Aβ40 and Aβ42 were significantly lower, compared to controls, and over time from diabetes diagnosis from adolescence to young adulthood.
Furthermore, changes in key plasma biomarkers of neurodegeneration from diabetes diagnosis to early adulthood have been correlated with worsening cognitive function in young adults with early-onset diabetes. These preliminary data suggest the possibility of an early risk trajectory for Alzheimer's disease among individuals diagnosed with diabetes during childhood or adolescence.
It is important to emphasize that the participants with youth-onset diabetes had lower plasma Aβ42 and Aβ40 concentrations than age-matched controls both in adolescence and early adulthood, suggesting amyloid dysregulation potentially early and sustained in diabetes beginning in young people. Lower levels of Aβ40, Aβ42 and their ratio, especially in plasma and cerebrospinal fluid, correspond to monomer sequestration and amyloid plaque formation. Overall, the lower plasma concentrations of Aβ42 and Aβ40 in their sample suggest the development of Alzheimer's disease neuropathology, but could also indicate disrupted neurodevelopment in those with early-onset diabetes.
NfL was not different from controls in adolescence, but was higher on average in the group of young adults with youth-onset diabetes, compared to young adult controls. These results are consistent with other larger studies in adults with diabetes. However, the scientists are cautious in interpreting their NfL results, given that NfL is a non-specific marker of neuronal damage and could also indicate involvement of peripheral neuropathy in people with diabetes. diabetes
The moral integrity of the authors of this study is reflected in the number of limitations highlighted by the authors. Too often, scientists (and their university's public relations department) make overblown, ridiculous, and deliberately misleading claims.
The young age of their sample limits confounding by age, such that changes in plasma biomarkers are more likely to be attributed to diabetes pathophysiology and not typical aging-related processes.
Their age-matched control groups were sampled from different cohorts. Thus, scientists cannot interpret biomarker differences between adolescent controls and young adult controls as a typical developmental change in these biomarkers. It should be noted that the shelf life of plasma samples from each group differed, which imposed yet another limitation on their study, with diabetes samples appearing in young people having on average a longer shelf life than control samples. .
Longer storage duration could impact the observed protein concentrations of the measured plasma biomarkers. However, if protein levels were differentially impacted between groups given variability in storage duration, we might expect to see lower concentrations in the youth diabetes group compared to control groups for all proteins measured. This was not the case in their study.
The scientists did not have APOE4 status among the subjects of their study.
The SEARCH study did not measure cognitive function at the initial visit so scientists could not study cognitive changes over time in relation to changing plasma biomarker levels.
Scientists do not have corresponding biomarkers measured in the CSF.
The sample of young adults with early-onset diabetes who participated in the PET imaging study was small.
In conclusion, although neurodegenerative diseases are conceptualized as a disease of the elderly, increasing evidence suggests that factors linked to early life may have an impact on risk trajectories.
Such life-span course disease models will contribute to a better understanding of how currently used neurodegeneartion biomarkers evolve during critical periods of development across the lifespan, and how they can be used to predict the risk of neurodegenerative diseases early onset and cognitive impairment in high-risk clinical populations such as early-onset diabetes.

 Sleep efficiency in older adults. Sleep deprivation increases amyloid-β (Aβ) concentrations in the interstitial fluid of experimental animal models and in cerebrospinal fluid in humans, while increased sleep decreases Aβ. Sleep abnormalities may therefore represent a risk factor for neurodegeneration.
Sleep efficiency in older adults. Sleep deprivation increases amyloid-β (Aβ) concentrations in the interstitial fluid of experimental animal models and in cerebrospinal fluid in humans, while increased sleep decreases Aβ. Sleep abnormalities may therefore represent a risk factor for neurodegeneration. These articles provide the reader with several points for further reflection.
- One of them is that the clinical trial cannot be the only method of validating the marketing of a drug. Indeed, for diseases that develop over decades, often asymptomatically, how can the effectiveness of a drug be tested? We can always dream of a pill that will cure in a few months a patient, as this is true for diseases where there is an identified pathogen, but it is very unlikely with chronic diseases. Even for communicable diseases, this type of medication does not always exist; a patient can be considered permanently cured, even though they suffer very serious after-effects.
These articles provide the reader with several points for further reflection.
- One of them is that the clinical trial cannot be the only method of validating the marketing of a drug. Indeed, for diseases that develop over decades, often asymptomatically, how can the effectiveness of a drug be tested? We can always dream of a pill that will cure in a few months a patient, as this is true for diseases where there is an identified pathogen, but it is very unlikely with chronic diseases. Even for communicable diseases, this type of medication does not always exist; a patient can be considered permanently cured, even though they suffer very serious after-effects. The exact reasons why atrial fibrillation (a common heart disease) increases dementia risk aren't fully understood, but there are likely several factors involved. Some of these factors directly cause damage, while others increase the chances of getting dementia over time. These factors include tiny blood blockages and bleeding in the brain's small blood vessels. Small strokes you might not even notice, reduce blood flow to the brain, increase long-term inflammation, and lead to shrinkage of brain tissue.
The exact reasons why atrial fibrillation (a common heart disease) increases dementia risk aren't fully understood, but there are likely several factors involved. Some of these factors directly cause damage, while others increase the chances of getting dementia over time. These factors include tiny blood blockages and bleeding in the brain's small blood vessels. Small strokes you might not even notice, reduce blood flow to the brain, increase long-term inflammation, and lead to shrinkage of brain tissue.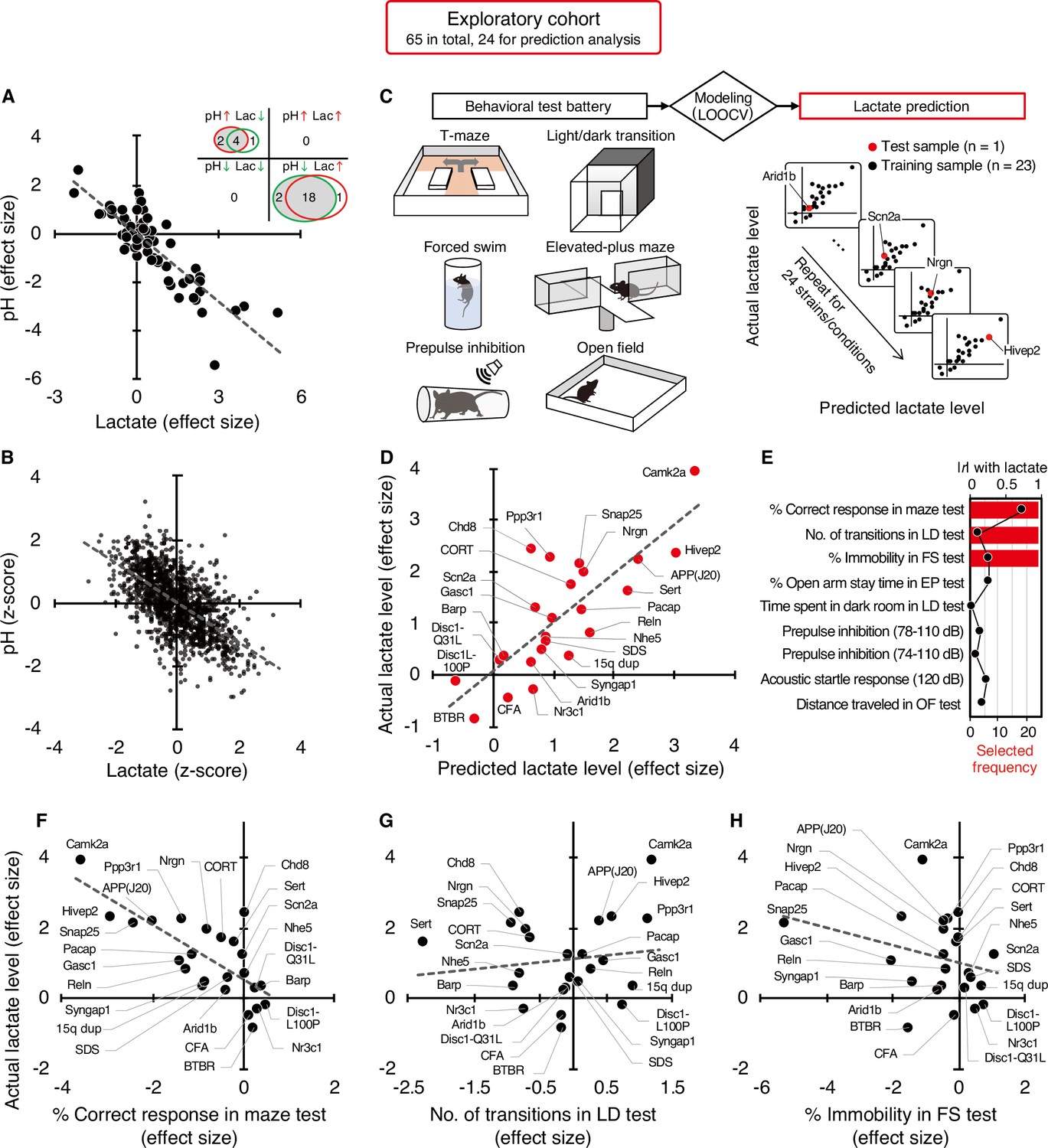 What could be the mechanism between increased lactate and decreased pH?
Lactate is a byproduct of metabolism and it is an acid, hence the acidification of pH. Neuronal activity relies heavily on glucose for energy. Under normal conditions, oxygen is readily available, and glucose is broken down through aerobic respiration, producing ATP (energy) with minimal lactate as a byproduct. However, during periods of high neuronal activity or limited oxygen supply, cells shift to anaerobic metabolism, relying more on glycolysis. This process produces more lactate as a byproduct.
What could be the mechanism between increased lactate and decreased pH?
Lactate is a byproduct of metabolism and it is an acid, hence the acidification of pH. Neuronal activity relies heavily on glucose for energy. Under normal conditions, oxygen is readily available, and glucose is broken down through aerobic respiration, producing ATP (energy) with minimal lactate as a byproduct. However, during periods of high neuronal activity or limited oxygen supply, cells shift to anaerobic metabolism, relying more on glycolysis. This process produces more lactate as a byproduct. Improving cognition using a simple probiotic would be an extraordinary result due to its simplicity of implementation.
Improving cognition using a simple probiotic would be an extraordinary result due to its simplicity of implementation.
 Studies have shown that oxidative stress and endoplasmic reticulum stress are correlated and can lead to protein misfolding (Abramov et al., 2020). Accumulation of misfolded proteins causes cellular damage and mitochondrial dysfunction and is associated with a range of neurodegenerative diseases, including ALS (misfolded SOD1, TDP-43, C9orf72) (McAlary et al., 2020), Parkinson's disease (misfolded α-synuclein) and Alzheimer disease (misfolded Aβ and Tau) (Abramov et al., 2020).
Studies have shown that oxidative stress and endoplasmic reticulum stress are correlated and can lead to protein misfolding (Abramov et al., 2020). Accumulation of misfolded proteins causes cellular damage and mitochondrial dysfunction and is associated with a range of neurodegenerative diseases, including ALS (misfolded SOD1, TDP-43, C9orf72) (McAlary et al., 2020), Parkinson's disease (misfolded α-synuclein) and Alzheimer disease (misfolded Aβ and Tau) (Abramov et al., 2020).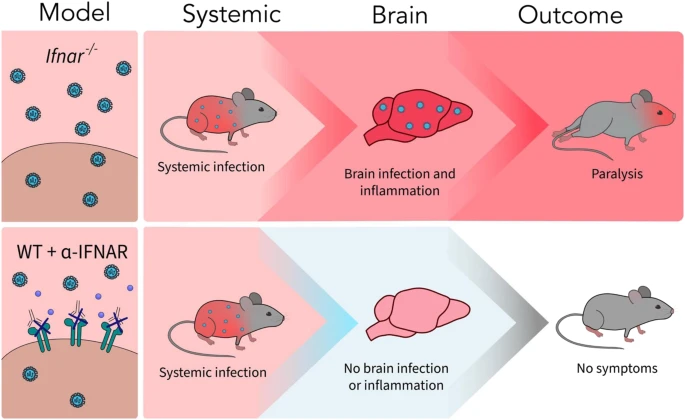 A new article aims to show that in the case of Zika viruses,
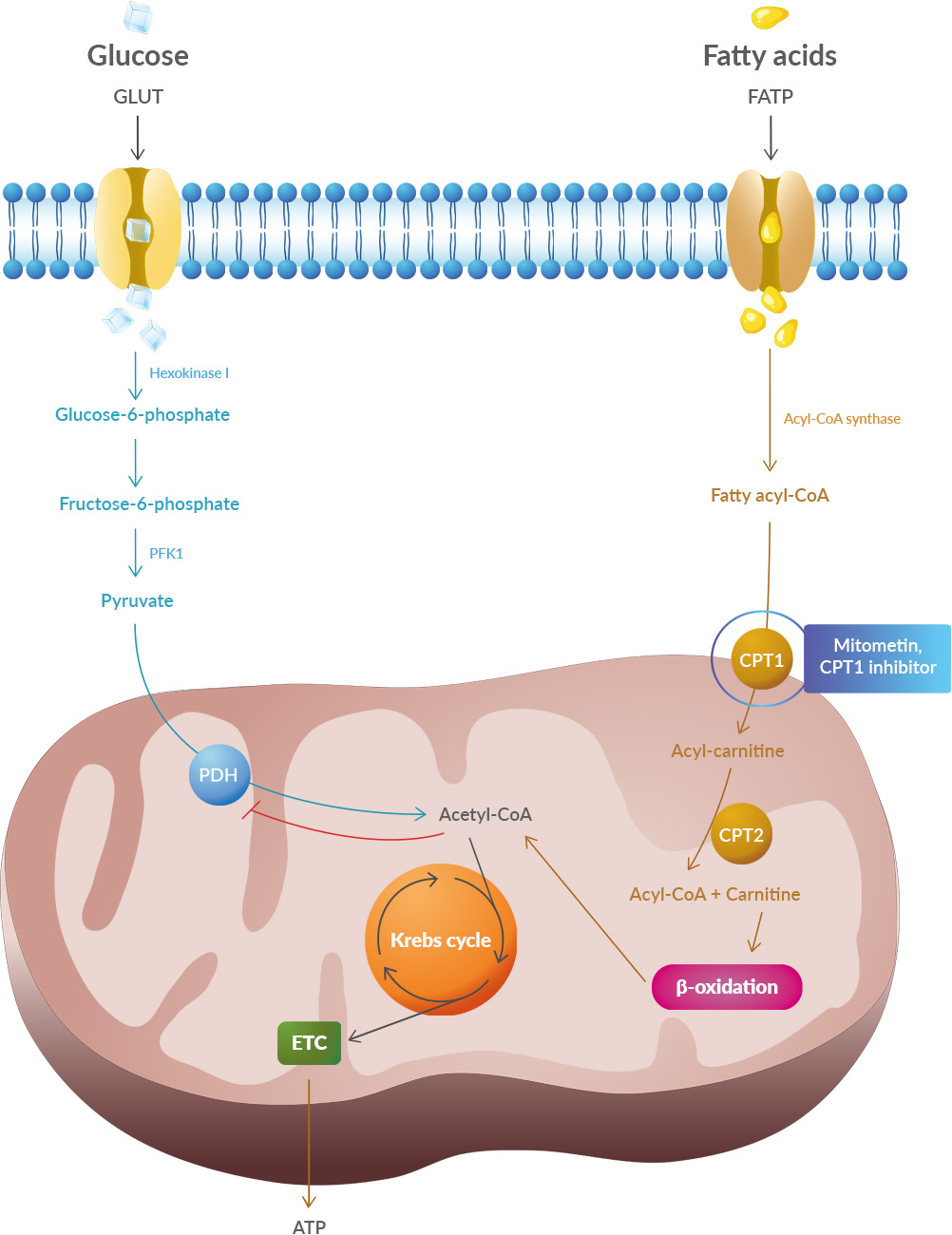
A new article aims to show that in the case of Zika viruses, 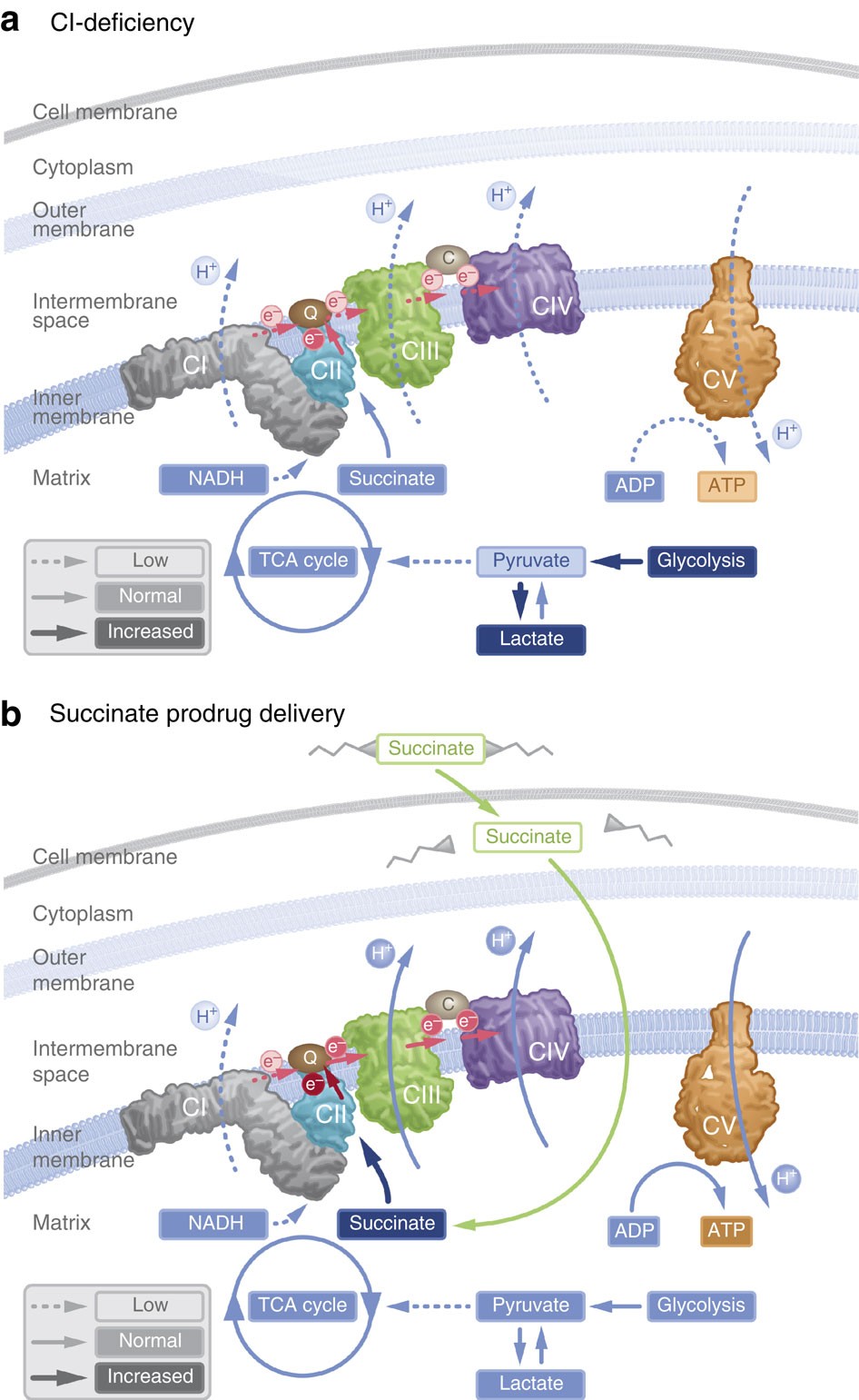 In particular, supplementation with dimethyl succinate, a substrate bypassing the inhibited step in the TCA cycle, suggests a potential therapeutic strategy to mitigate mitochondrial dysfunction in Alzheimer's disease. Dimethyl succinate (DMS), a membrane-permeant form of succinate, could serve as a pro-drug to provide substrate to the next enzymatic step in the TCA cycle, succinate dehydrogenase (SDH).
In particular, supplementation with dimethyl succinate, a substrate bypassing the inhibited step in the TCA cycle, suggests a potential therapeutic strategy to mitigate mitochondrial dysfunction in Alzheimer's disease. Dimethyl succinate (DMS), a membrane-permeant form of succinate, could serve as a pro-drug to provide substrate to the next enzymatic step in the TCA cycle, succinate dehydrogenase (SDH).