This post is about a review of the contribution of Advanced Glycation Endproducts to Neurodegenerative Diseases.
Advanced Glycation Endproducts (AGEs) are formed through non-enzymatic reactions between proteins, aminoglycosides, amino-terminal lipids, and reducing sugars like D-glucose. This process involves Amadori rearrangements and oxidative modifications. The accumulation of AGEs, especially under conditions of elevated oxidative stress, leads to various diseases.
 AGEs have diverse structures, but only a limited number have been characterized. Some AGEs are small molecules formed through proteolytic degradation of protein-crosslinked or protein-modified AGEs. Imbalance between the formation and destruction of AGEs, particularly under conditions of oxidative stress, results in excessive accumulation and disease progression.
AGEs have diverse structures, but only a limited number have been characterized. Some AGEs are small molecules formed through proteolytic degradation of protein-crosslinked or protein-modified AGEs. Imbalance between the formation and destruction of AGEs, particularly under conditions of oxidative stress, results in excessive accumulation and disease progression.
Some of the well-characterized AGEs include pentosidine, glucosepane, Argpyrimidine, and Nε-(carboxymethyl)lysine (CML). The imbalance between the formation and destruction of AGEs, triggers a cascade of signaling events, inflammation, and oxidative stress. This inflammatory signaling cascade is associated with various neurological diseases, including Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), diabetic neuropathy, diabetes, and atherosclerosis.
Other endogenous ligands are also involved, such as high mobility group box1 (MGB1) proteins. Additionally, exogenously ingested AGEs contribute to disease onset. The formation and accumulation of AGEs overwhelm the body's detoxification mechanisms under conditions of enhanced oxidative stress, exacerbating neurodegenerative diseases and other inflammatory-associated conditions.
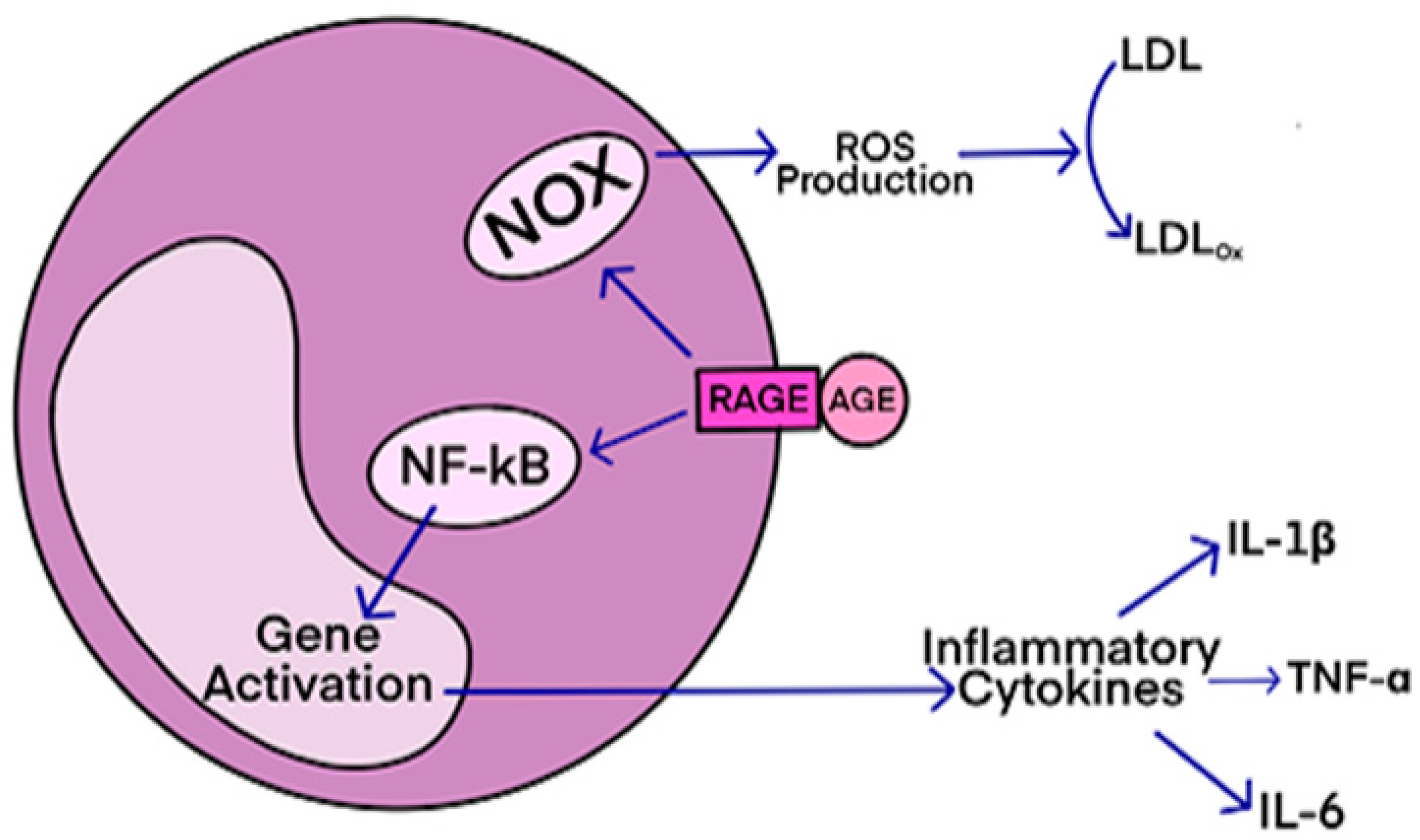
RAGE (receptor for advanced glycation end-products) is a receptor that interacts with AGE (advanced glycation end-products)-derived ligands, such as CML, CEL, and MG-H1. The ligands bind to specific residues on the receptor, initiating signal transduction and pro-inflammatory signaling events.
RAGE antagonists, either endogenous or exogenous compounds, bind to RAGE and attenuate the binding interactions between AGE and RAGE, thereby preventing disease progression. These antagonists have shown potential for treating neurodegenerative diseases, diabetes, atherosclerosis, and cancers. Some small molecule-based RAGE inhibitors, like FPS-ZM1 and Azeliragon, have entered clinical trials, but none have been FDA-approved yet.
FPS-ZM1 has shown selective binding to RAGE and inhibits the formation of Aβ peptides, which are associated with brain damage in diseases like Alzheimer's. RAGE inhibitors also have potential therapeutic applications in diabetic nephropathy, cancer cell metastasis, and Parkinson's disease.
Azeliragon, currently in phase 3 clinical trials, has demonstrated decreased levels of Aβ plaques in the brain, reduces inflammation, and slower cognitive decline in Alzheimer's patients. Other compounds, such as urolithin and its analogs, have shown comparable RAGE inhibition to Azeliragon.
RAGE antagonists are also considered as therapeutics for diabetic neuropathy and retinopathy. Inhibitors of the cytoplasmic tail of RAGE (ct-RAGE) have been investigated and shown to block AGE/RAGE-mediated signaling events effectively. Some of these small molecule antagonists have structural similarities to FPS-ZM1 and have demonstrated potential for treating neurological disorders and diabetic complications.
Dietary AGEs, especially from animal-derived foods cooked at high temperatures, can also contribute to AGE accumulation and the development of diseases. RAGE antagonists, AGE inhibitors, and soluble RAGE (sRAGE) have shown promise in the treatment of age-related pathologies. Polyphenolic compounds can attenuate AGE formation and its toxic effects by reducing oxidative stress.
Therapeutic interventions targeting the AGE-RAGE axis may provide effective treatment for neurodegenerative diseases, diabetes, atherosclerosis, and cancers.
In summary, RAGE antagonists show promise as therapeutics for various diseases by attenuating the binding interactions between AGE and RAGE and preventing downstream pro-inflammatory signaling events. Clinical trials are underway for several RAGE inhibitors, and they have shown potential for treating Alzheimer's, diabetes, and other AGE-related diseases.
Overall, understanding the formation, toxicity, and interactions of AGEs with RAGE is crucial for developing therapies to combat age-related diseases.
