Therapeutic hypothermia is now the standard of care for a variety of conditions involving cell death.
The mechanisms of action of therapeutic hypothermia are varied and poorly understood. They include mechanisms that are thought to be also involved in ALS.
However, the possibility that therapeutic hypothermia has an application in ALS had not been examined.
Lee J Martin, Mark V Niedzwiecki and Margaret Wong, the authors of the article reviewed today, therefore tested in a transgenic mouse model of ALS the hypothesis that chronic intermittent mild hypothermia has therapeutic efficacy.
Mice were observed twice and then 4 to 5 times per day in the terminal stage of the disease.
SOD1 mouse models are chronically feverish. At 6 weeks of age, the internal temperature of model SOD1 mice was significantly higher (38 ° C) compared to non-transgenic control mice (37 ° C).
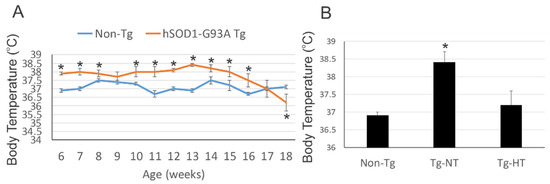
At 8 weeks of age, SOD1 mice were randomized into several groups. The SOD1 mouse model had a body temperature 1 to 2 ° C lower than the SOD1 mouse model not undergoing cooling.
The precooling body temperatures among the SOD1 mouse model groups did not differ significantly. Warming of the mouse was slow and spontaneous at room temperature. At room temperature, motor activity was tested on a racing wheel with voluntary activity.
The onset of the disease was assessed quantitatively by a deficit of activity and descriptively by paresis of the hind limbs. To assess the effectiveness of hypothermia, a group of mice were euthanized before the final stage at 12 weeks of age.
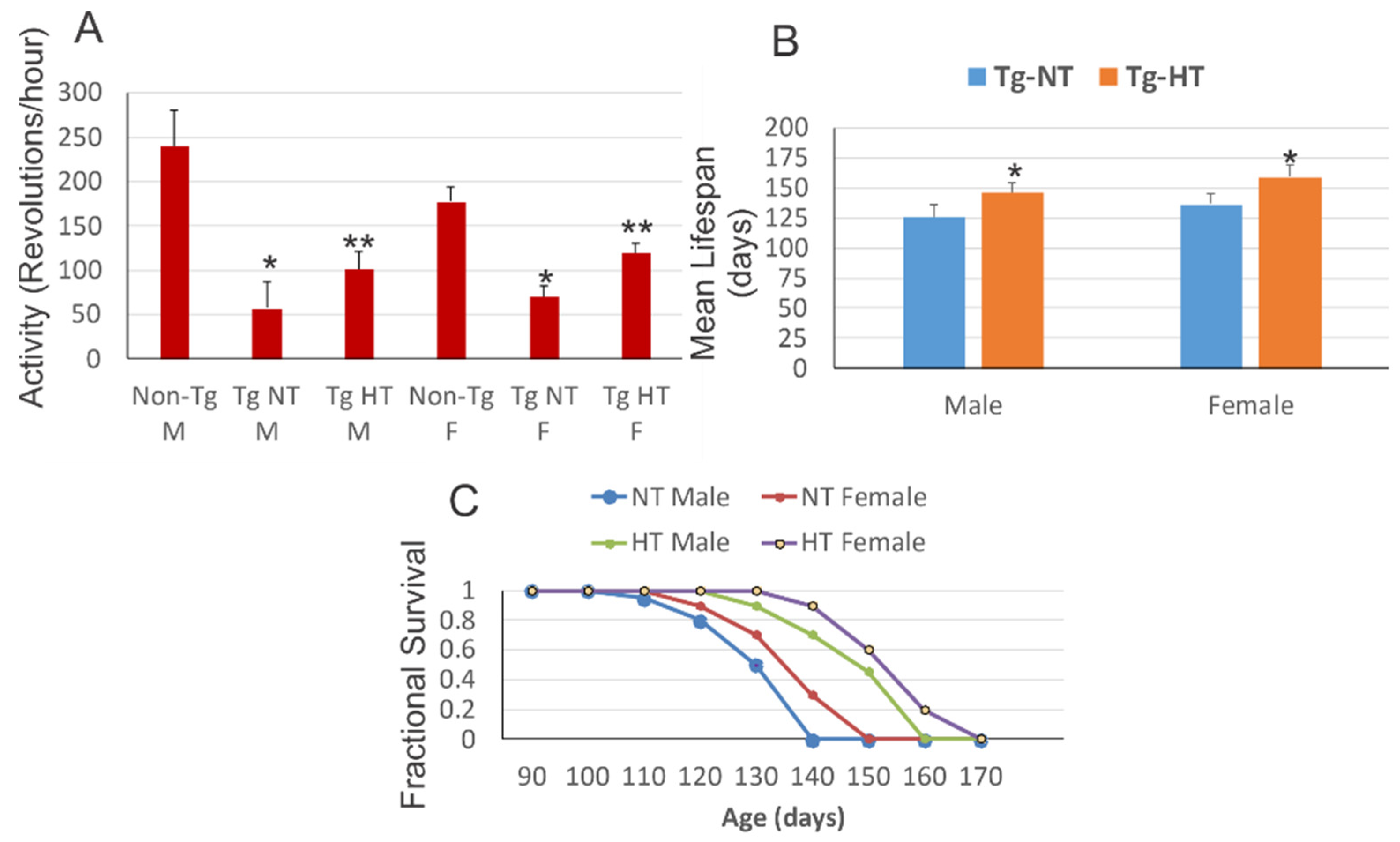
At 10 weeks of age, hypothermia improved neurological outcomes and survival in the SOD1 mouse model. Male and female SOD1 mouse models housed at room temperature exhibited a significant reduction in motor activity compared to sex-matched non-transgenic littermates. Cold acclimatized male SOD1 mice and female SOD1 mice showed significantly improved motor activity compared to the SOD1 mouse model maintained at normal temperature.
However, all groups of SOD1 mice exhibited significant motor deficits compared to non-transgenic mice of the same age. The mean lifespan of the cold acclimatized male SOD1 mouse model was significantly increased compared to the mouse model exposed to normal temperature. Cold acclimatization of the female SOD1 mouse model obtained a slightly better result on the significant increase in lifespan compared to the female SOD1 mouse model at normal temperature. 3.4.
The authors assessed hypothermic, normal-temperature SOD1 mice at 12 weeks of age for spinal cord neuropathology. Non-transgenic mice had evident spinal motor neurons with large multipolar cell bodies. In uncooled SOD1 mice, motor neurons were significantly 75% depleted and there was a secondary fulminant infiltration of small cells into the parenchyma. In cold acclimatized SOD1 mice, motor neurons were more apparent than uncooled mice.
Hypothermia rescued motor neuron counts in the lumbar spinal cord of the SOD1 mouse model (40% loss) compared to 80% loss in the uncooled SOD1 mice. Secondary small cell inflammation of the parenchyma of the spinal cord appeared to be reduced in cold acclimatized mice.
In uncooled SOD1 mice, the mitochondria were severely swollen, dysmorphic, and disrupted in motor neurons and in the neuropil. In hypothermic SOD1 mice, the mitochondria in the cell bodies of motor neurons were protected from swelling. This protection of motor neurons in SOD1 mice has been linked by an attenuation of inflammatory changes in the spinal cord.
In non-transgenic mice, diaphragm motor plate innervation was close to 100%, while in uncooled SOD1 mice, endplate innervation was significantly reduced to only about 40% at 12 weeks old.
In contrast, in cold acclimatized SOD1 mice, innervation of the neuromuscular junction was restored to about 65% of normal, but was still significantly reduced compared to diaphragm innervation from non-transgenic mice.
The cooled SOD1 mice exhibited a significant upregulation of the modified proteins HSP70, UCP3 and SUMO1 compared to the uncooled transgenic mice.
Cold acclimatization was more effective in females than in males at prolonging lifespan. The innervation of the diaphragm of the motor plates was improved by hypothermia.
Many circulating cytokines induce fever. This profile of inflammatory cytokines is consistent with their finding that ALS mice are febrile during the course of the disease. The main circulating proinflammatory pyrogenic cytokines in ALS mice are TNFα and IL6. TNFα and IL6 can cause muscle atrophy in various clinical settings. Skeletal muscle wasting is an important feature of human ALS and some mouse models of ALS.
The authors confirmed, which has been repeatedly reported, that during the course of the disease in the muscles of SOD1 mice, nitric oxide production is increased and protein nitration is elevated, y including key proteins at the neuromuscular junction.
Nitric oxide is an essential regulator of cell apoptosis. It can have an antiapoptotic effect, or, conversely, an apoptotic effect. This rocker is intimately linked to the presence or absence of cellular reducers such as glutathione.
In ALS, the thermoregulatory function could be aberrant. Hypothermia could act on the CNS, peripheral nervous system, skeletal muscle and body fat levels.
The clinical-translational application and efficacy testing of this concept in human ALS would be non-invasive and applicable, perhaps in rehabilitation spas in private and hospital settings. Cold water immersion or cryotherapy is common among athletes. Cold acclimatization protocols should be determined and empirically refined, and biomarkers of therapeutic efficacy should be identified for human ALS. Biomarkers could be based on skeletal muscle biopsy and assay for HSP70, protein sumoylation, and mPTP activation thresholds.

