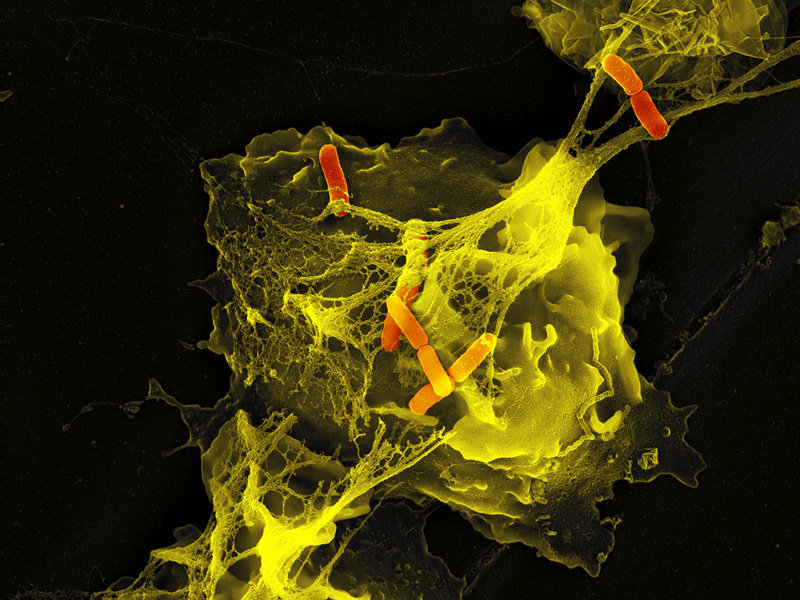
Neutrophils are the immune system's first line of defense against infection and are generally thought to kill invading pathogens using two strategies: engulfing microbes and secreting antimicrobials. In 2004, a new function was identified: the formation of NET NETs are very fine filaments visible in scanning electron microscopy. The fibers are decorated with globular particles and grouped together to form more complex structures.
 Source: Max Planck institute for infection biology
Source: Max Planck institute for infection biology
NETs allow a high local concentration of antimicrobial components and bind, disarm and kill not only bacteria but also pathogenic fungi. In addition to their antimicrobial properties, NETs can serve as a physical barrier that prevents the further spread of pathogens.
NETs have been shown to form in blood vessels, particularly in the pulmonary capillaries and hepatic sinusoids. Intravascular NET formation is tightly controlled and regulated by platelets, which detect severe infection and then bind to neutrophils and activate them to form NET. Platelet-induced NET formation occurs within minutes and NET can then intercept circulating bacteria as they pass through the vessels.
NETs could however have a deleterious effect on the host, since the extracellular exposure of histone complexes could play a role during the development of many diseases.
The authors of a study published in Nature Immunology, have identified an intrinsic cellular program which modifies the proteome of neutrophils and causes the progressive loss of the granule content and the reduction of the formation capacity of NET.
https://www.ncbi.nlm.nih.gov/pubmed/31932813
It has long been recognized that multiple inflammatory processes in humans exhibit circadian periodicity, and experimental models have supported that not only the onset of inflammation, but also the severity of inflammatory events manifest diurnal oscillations. These events are often caused by activation of neutrophils and thrombosis, which in turn can be exacerbated in the presence of neutrophils or NET. Thus, the authors' results, which are obtained in the context of acute pulmonary inflammation, may extend to other inflammatory and thrombotic conditions.
Proteome changes are driven by CXCR2 and Bmal1. The diurnal changes in neutrophil transcription and migration are controlled by an intrinsic cellular mechanism, in which the expression of the chemokine Cxcl2 is regulated by the molecular clock protein Bmal1 and leads to the autonomic diurnal activation of neutrophil cells by signaling via CXCR2.
The intrinsic cellular program which modifies the proteome of neutrophils in the bloodstream, also causes the gradual loss of the granule content and the reduction of the NET formation capacity.
Changes in the proteome, granule content, and NET formation thus indicate a possible strategy for "disarming" neutrophils.
